What's Nucletouch?
News (2021/01/05)
- THE MID-JAPAN ECONOMIST (2020/07/25)
- A special elemental magic - Kyoto scientists announce a 'nuclear' periodic table(EurekAlert 2020/05/28)
- Springer Nature Twitter (2020/05/21)
- UNIVERSITY JOURNAL ONLINE (2020/05/10)
- Nihon Keizai Shimbun (2020/05/03)
- NIKKAN KOGYO SHIMBUN (2020/04/23)
- The Yomiuri Shimbun (2020/04/22)
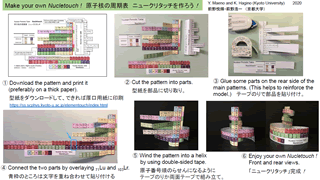
[Free download] Download the pattern to make your own Nucletouch.
Click the image to see a larger version.
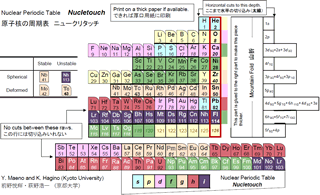
Periodic Table of Atomic Nuclei "Nucletouch"
Overview
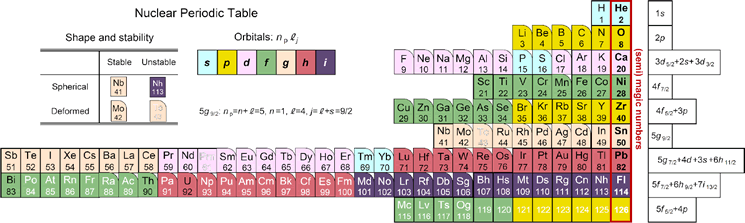
First look at the new periodic table below. It is similar to the periodic table of elements that appears in junior high school textbooks, but the arrangement of element symbols is different. The order of Sui, He, Lee, Be (Hydrogen H, Helium He, Lithium Li, Beryllium Be) is the same, but under helium, oxygen, calcium, etc., instead of the rare gas stabilizing elements neon, argon, etc. Are lined up.
Actually, this is the periodic table of "nuclei". It is well known that the nucleus becomes stable when the number of protons contained in the nucleus becomes 2, 8, 20, 28 ... called the magic number. Surprisingly, however, I haven't found an example of it in the form of a periodic table. This new periodic table was devised by Professor Koichi Hagino and Yoshiteru Maeno of the Graduate School of Science, Kyoto University.
This achievement was published in the academic journal Foundations of Chemistry (April 21, 2020) published by Springer Nature.
*Click the image to see a larger version.
1. Background
At the center of the atoms that make up an element is an atomic nucleus, around which electrons circulate (Fig. 2). The number of protons contained in the nucleus is the "atomic number". The "Periodic Table of Elements" expresses that elements with similar properties appear periodically by arranging the element symbols in the order of atomic numbers (Fig. 3). Last year 2019 was the 150th anniversary of Dmitri Mendeleev's publication of the Periodic Table based on the discovery of the Periodic Table of the Elements, and was expanded around the world as the UNESCO International Periodic Table Year. Currently, the periodic table that is widely used all over the world is actually based on the "long periodic table" devised by Werner, not the "short periodic table" devised by Mendeleev.
Figure 2: Schematic diagram of the structure of atoms and nuclei
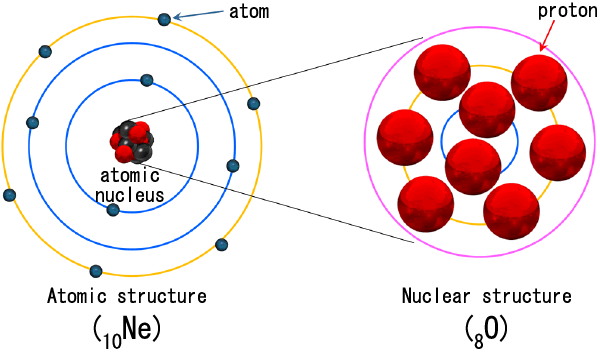
Figure 3. Periodic Table of the Elements (Werner's Periodic Table of the Period)
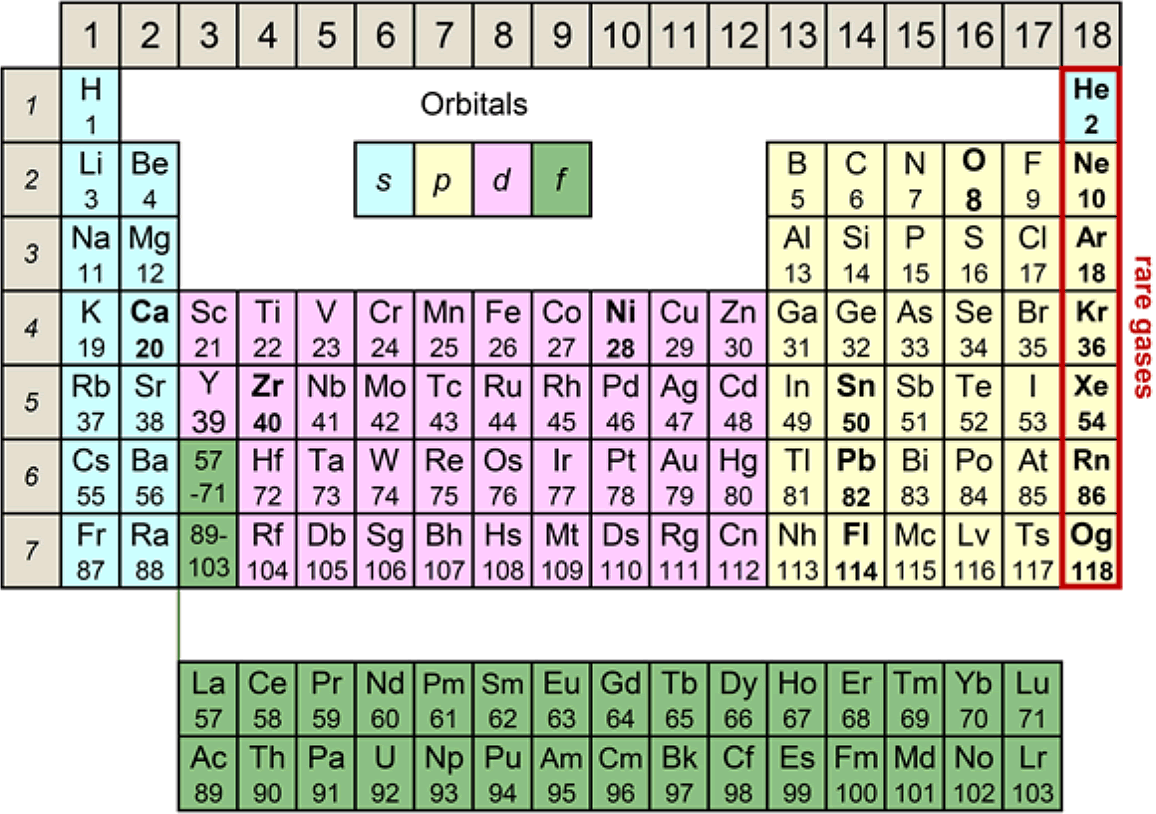
The nucleus has positive electricity proportional to the number of protons. The Periodic Table of the Elements expresses how the electrons that go around it are clogged in order from the orbit with the lowest energy. The colors in Figure 3 show the difference in orbit. Helium, neon, etc. in the rightmost column are called rare gases, and because the electrons have just filled the shell of the orbit, they are very stable elements that do not cause a chemical reaction.
In fact, we can think of similar orbital motions for the protons and neutrons that make up the nucleus itself. There is no other "nucleus" at the center of the nucleus. However, due to the interaction that acts on protons and neutrons (strong interaction called "nuclear force" that Hideki Yukawa considered), orbits that are divided into several shells like electrons are formed, and the orbits are clogged in order from the lowest energy orbit. I will continue. This is called the nuclear shell model (Karamokei, shell model) (Note). The magic number nucleus becomes a nucleus whose shell is just buried, and corresponds to rare gas. However, since the environment (potential) of electrons and protons / neutrons is different as shown in Fig. 2, the maximum number of particles that each shell can accommodate differs greatly between the case of atoms and the case of atomic nuclei. In the case of atoms, the number of electrons is 2, 10, 18, 36, 54, 86, but in the case of atomic nuclei, the number of protons is 2, 8, 50, 82, It's time to reach 114. Also, when the number of protons reaches 40, it shows similar properties to a lesser extent. Neutrons have a similar magic number, which is the same as protons, except that it is 126 instead of 114.
2. Ideas and achievements
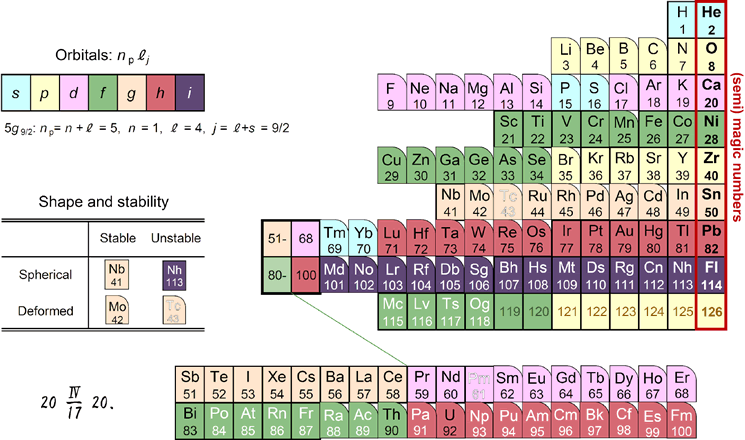
Then, is it possible to make a periodic table of the nucleus? Since the nucleus contains neutrons in addition to protons, there are isotopes with different numbers of neutrons even for the same element. The number of neutrons also greatly affects the stability of the nucleus. A nuclear chart that covers all of them is known, but the correspondence with the periodic table of elements is not visible. Therefore, by selecting the most representative isotopes for each element, we devised a periodic table of nuclei corresponding to the periodic table of the elements introduced in Fig. 1 at the beginning. At the right end, elements whose number of protons is a magic number are lined up corresponding to the rare gas. Curiously, such a periodic table of nuclei has never been known and is the first in the world.
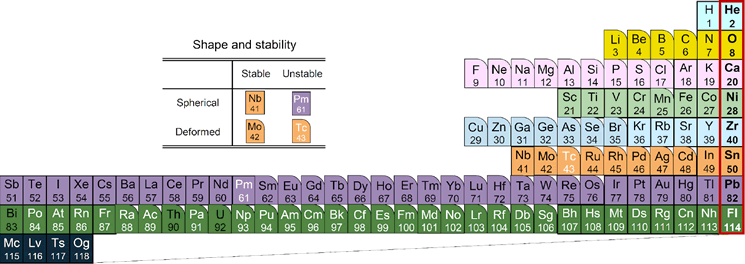
Figure 4 below shows the orbital shells of protons color-coded according to the Periodic Table of the Elements (Fig. 3). You can also visually understand the difference between the magic number of atoms and the magic number of atomic nuclei. The blue background color represents the s orbital (the blue circle in Fig. 2) that spreads out in a circle. The shell consisting of the third 3s orbit is filled with magnesium (Mg), which is the 12th element for electrons, but ytterbium (Yb), which is the 70th element for protons, reflecting the large difference in the properties of the orbits of electrons and protons. It has been.
*Click the image to see a larger version.
Looking at this periodic table, stable nuclei that satisfy magic numbers have a shape close to a sphere. On the other hand, the farther away from the magic number, the more deformed nuclei (boxes with rounded corners) like rugby balls, and the unstable nuclei that spontaneously collapse even if the atomic number is smaller than lead (Pb) (Pb). White element symbol) will appear. In this way, in this new periodic table of nuclei, not only the shell structure but also the deformation and stability of the nuclei of each element can be seen at a glance. Furthermore, Fig. 5 shows a shape similar to the frequently used elemental periodic table of atoms in Fig. 3. There is a big difference to note here. In the periodic table of atoms, the upper and lower elements belonging to the same column (group) have similar properties because they have electrons of the same orbit as shown in the same color. However, in the periodic table of nuclei, although there is a periodicity of properties around magic numbers as described above, as you can see from the difference in color, they have different orbital protons above and below the same column, so those properties There is no particular similarity to.
*Click the image to see a larger version.
3. the next deployment
By creating the periodic table of prokaryotes, which can be easily compared with the periodic table of elements, we can create a new index for learning the properties of prokaryotes, including magic numbers. We hope that the periodic table of prokaryotes will be published in university textbooks in the future so that it can be used in education.
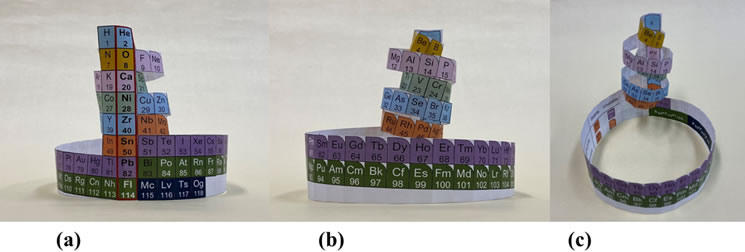
The periodic table of prokaryotes can also be created physically. You can easily make the model shown in the photo below (Fig. 6) by printing Fig. 1 (Fig. 2 of the publication theory) on paper and using scissors and cellophane tape. As for the periodic table of physical elements, "Element Touch", which Maeno devised about 20 years ago, is known and is also sold as Kyoto Institute of Science goods. Correspondingly, the periodic table of prokaryotes (nuclei) has been named "Nucleic Touch".
*Click the image to see a larger version.
4. Supported research projects
We received support from the following projects for the activities of "Science and Technology Dialogue with the National Government".
- Japan Society for the Promotion of Science Base Formation Project (A: Advanced Base Formation) (Representative: Yoshiteru Maeno) (2017-2021).
- Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research / Foundation S (Representative: Yoshiteru Maeno) (2017-2021, No.JP17H06136).
- Japan Society for the Promotion of Science New Academic Area Research / Planning Research (Representative: Yoshiteru Maeno) (2015-2019, No.JP15H05852).
Explanation of words
(Note) Shell model (Karamokei, shell model):In 1949, Goeppert Mayer and Jensen independently performed the magic numbers of prokaryotes from the perspective that each nucleus is independently moving within the average potential (mean field) created by other nuclei. I succeeded in explaining. It was shown that strong spin-orbit force plays an essential role. For this achievement, the two were awarded the 1963 Nobel Prize in Physics. So far, three women have won the Nobel Prize in Physics, and Goeppert Mayer was the second.
Discussion title and author
A Nuclear Periodic TableKouichi Hagino and Yoshiteru Maeno
Foundations of Chemistry
DOI:https://doi.org/10.1007/s10698-020-09365-5
(Anyone can download it for free.)