What's Elementouch?
What is the difference with the conventional periodic table?
"Elementouh" is a name that has two meanings: "touch the element" and "standing in Japanese". By capturing the periodic table in three dimensions, the nature and regularity of elements are expressed more accurately, and the world of elements that can be visually understood is expanded.
Periodic table history
The basic ingredients that make up a substance are called "elements." For example, hydrogen, oxygen, calcium, iron, etc. are the elements, but there are only over 100 kinds of elements in the world. In other words, everything around us, living things, the earth, and the stars are all made up of a combination of just over a hundred elements. This fact alone is enough to remind us of the depth of nature's providence. The periodic table of elements, which summarizes the regularity of the elements in a single table, is a very basic tool for all natural sciences.
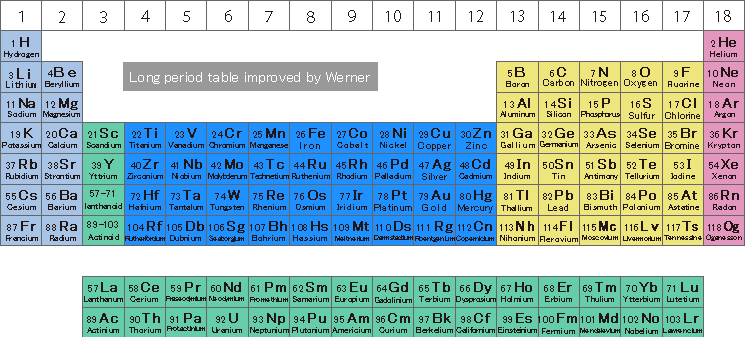
The currently widely used periodic table starts from the short periodic table devised by Mendeleev in 1869, It is a "long period table" that was said to have been improved by Werner (who won the Nobel Prize in Chemistry in 1913) in 1905 (see figure below). The fact that this table was invented about 100 years ago is a wonderful achievement, but it is also absurd. Then, as we entered the 21st century, this three-dimensional periodic table Elementouch was created by breaking down the conventional periodic method from a new perspective.
*Click the image to see a larger version.
Features of Elementouch
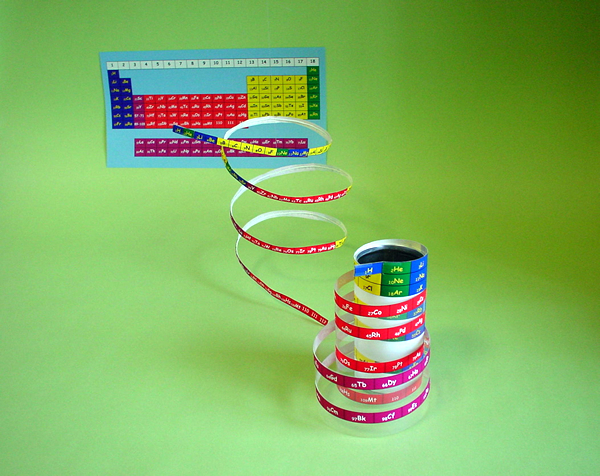
The long periodic table is the most complete periodic table, but it is not perfect yet. Elementouch is a new periodic table in which the symbols of elements are arranged in a spiral pattern, and we have succeeded in solving the problems of the long periodic table.
1. All elements are arranged neatly in numerical order!
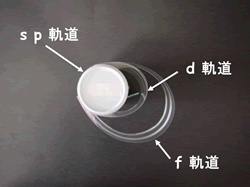
An element is a small particle with a size of one hundred millionth of a centimeter, and this particle is called an "atom". At the center of the atom is a core called the "atomic nucleus," around which a number of "electrons" orbit. There are four types of orbits, s, p, d, and f, depending on the difference in the momentum (angular momentum) of electrons. The orbits of the outermost electrons (outermost shell electrons) play an important role in determining the properties of each element.
"12Mg" and "13Al" are similar in nature and should be lined up next to each other, but they are very far apart. The number at the bottom left of the element symbol is called the atomic number, which represents the number of protons contained in the nucleus.) This is a problem that occurred because there are elements called d-electron transition metals from groups 3 to 12 in the 4th row and below. Also, in the long periodic table, there are many elements that are protruding from the small table below.
d-electron transition metal elements (such as "21Sc" to "30Zn") have d-electrons in the outermost shell, and these are incorporated in the center of the periodic table. However, since the f-electron transition metal element j is treated as a separate table below, the atomic numbers "56Ba" and "72Hf" are separated. Unlike when the long periodic table was created, the lanthanoid elements, which are f-electron transition metal elements, are now extremely close to demand and are now familiar elements, including magnetic materials that support the high-tech industry. It should not be treated. In Element Touch, all elements start from "1H" and are arranged in a continuous spiral in atomic symbol order.
2. The electron orbit is also represented!
3. At a glance you can see the properties of the elements!
Do you know the phenomenon of superconductivity in which electric current flows without energy loss? Technology is expected to expand in practical use in the 21st century, but the key to this is the development of new materials. In doing so, it is important to know what elements have similar properties. New materials are often found by replacing elements that are distant in the long periodic table with elements that line up in the same column in element touch.
For example, a new superconductor was discovered by replacing "20Ca" with "48Cd". Both of them tend to become +2 valent ions, and the "group 2" and "group 12" to which these elements belong were called "group 2A" and "group 2B" before 1985, respectively. Moreover, in Mendelev's short period table, these were originally in the same row. This was because the metal element expressed the most stable electron valence when forming an oxide. In addition to this, group 22 "22Ti" and group 14 "50Sn", which are prone to +4 nonmagnetic ions, are also unrelated positions in the long periodic table.
Of the rare earth elements including lanthanoids, "71Lu" is very similar to "39Y" and "57La" in that it becomes a trivalent non-magnetic ion, but it was not clear in the long periodic table.
In Elementouch, elements that tend to be divalent, trivalent, or tetravalent are now lined up vertically in response to these problems. Furthermore, the difference between the typical element and the transition metal element is expressed by the difference in color. In the example above, "Ca" and "Cd", "Y" and "La" and "Lu", "Ti" and "Sn" are now aligned vertically (bottom photo).
*Click the image to see a larger version.