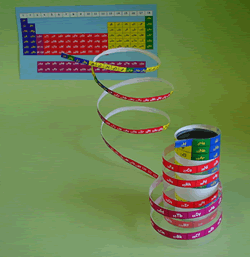
What's Elementouch?
What is the difference from the conventional periodic table?
Element Touch (Elementouh) is a name created by adding the meaning of "touch" to "element" and the meaning of "standing".
By capturing the periodic table in three dimensions, the nature and regularity of elements are more accurately represented, and the world of elements that can be understood visually has been expanded.
![]() You can see 3D Element Touch commentary on YouTube.
You can see 3D Element Touch commentary on YouTube.
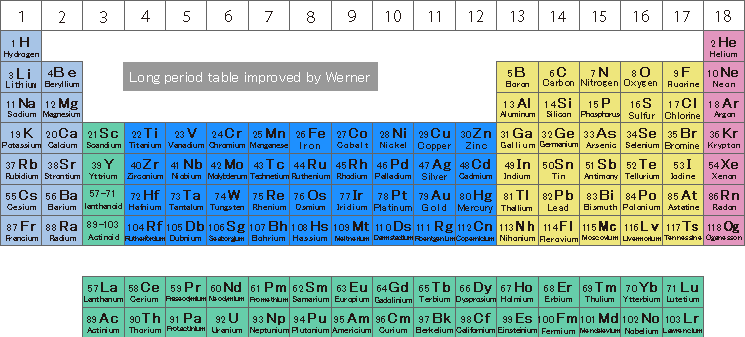
History of Periodic Table of Elements
*Click on the image to enlarge it.
For example, 20Ca and 48Cd, Group 4 22Ti and 50Sn are in seemingly unrelated positions in the long period table. Also, 71Lu at the right end of the table below is very similar to 39Y and 57La, but it is not clear in the long period table. In the element touch, Ca and Cd, Ti and Sn, and Y, La and Lu are now aligned in a row.
Element touch features
The long periodic table is the most complete periodic table, but it is still not perfect. Element Touch is a new periodic table in which elemental symbols are arranged in a spiral, and succeeded in solving the problems of the long periodic table.
1.All the elements are neatly arranged in numerical order!
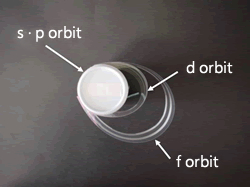
The identity of the element is a small particle about 100 millionths in size, and this particle is called an atom. At the center of an atom is a core called a nucleus, around which a number of electrons circulate. There are four kinds of trajectories, s, p, d and f, depending on the difference in the momentum of the electron (angular momentum). And the orbit of the outermost electron (the outermost electron) plays an important role in determining the properties of each element.
Well, the first problem of the long periodic table is that the elements are not arranged seamlessly. The properties of magnesium 12 Mg and aluminum 13 Al are similar, and there is a lot of sense of the elements that should be aligned next to each other from the atomic number. (The number at the bottom left of the symbol is called the atomic number and indicates the number of protons contained in the nucleus.)
This is an element called d-transition metal from Group 3 to Group 12 in the fourth line and below It is unreasonable to have occurred. In the long periodic table, there are many elements that have been exposed in the small table below.
d Electron transition metal elements (such as scandium 21Sc to zinc 30Zn) have d electrons in the outermost shell, and these are incorporated in the center of the periodic table. However, the f-electron system transition metal element j is treated as a separate table below, so there is a discontinuity between barium with atomic number 56 and hafnium with 72. Unlike when the long period table was created, lanthanide elements, which are f-electron transition metals, are now very popular elements in demand, including magnetic materials that support the high-tech industry. It should not be treated. In the element touch, starting with hydrogen of atomic number 1, all elements are arranged in a spiral without breaking in atomic symbol order.
2.It also expresses the electron orbit around the nucleus!
3.At a glance you can see the nature of the elements in the compound!
Do you know the phenomenon of superconductivity where current flows without energy loss? In the 21st century, the technology is expected to be expanded for practical use, but the key to holding it is the development of new materials. At that time, it is important to know what kind of elements have similar properties. In fact, new elements can often be found by replacing elements with the same vertical row in the Elemente Touch, although they are quite far away in the conventional long-period table.
For example, a new superconductor was discovered by replacing 20Ca with 48Cd. Both tend to be +2 ions, and groups 2 and 12 to which these elements belong were called groups 2A and 2B before 1985, respectively. What's more, in Mendeleef's short periodic table, these were originally in the same column. This was because the metal element expressed the most stable valence number when forming an oxide. Besides this, Group 4 22Ti and Group 14 50Sn, both of which are likely to be +4 nonmagnetic ions, are also seemingly unrelated positions in the long period table.
In addition, lutetium 71Lu with atomic number 71 is similar to 39Y and 57La among the rare earth elements including lanthanides in that it is a trivalent nonmagnetic ion, but it was not clear in the long periodic table.
In response to these problems, elements that are likely to be divalent, trivalent, and tetravalent are now aligned vertically in Elementen Touch. At the same time, the difference between the typical element and the transition metal element is represented by the difference in color. In the example above, Ca and Cd, Ti and Sn, and Y, La and Lu are aligned in a row (Please see below.).

Divalent element series
Ca and Cd are aligned.
.gif)
Trivalent element series
Y, La, Lu in the same column

Tetravalent element series
Ti and Sn in the same column